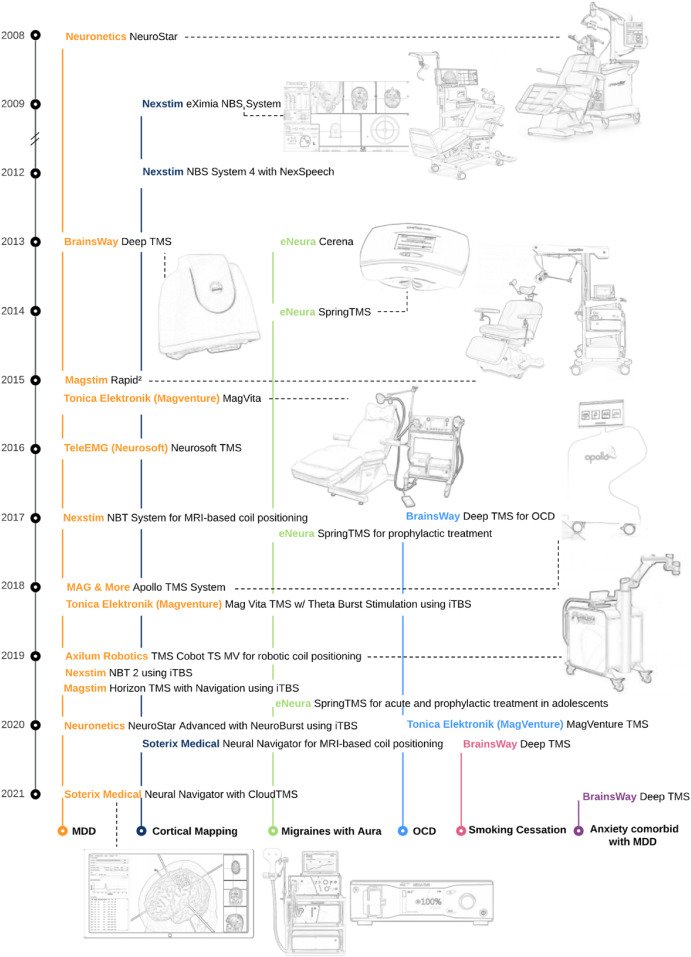
A Visual and Narrative Timeline of US FDA Milestones for Transcranial Magnetic Stimulation (TMS) Devices
Full Paper Link Here: Cohen et al 2022
A visual and narrative timeline of US FDA milestones for Transcranial Magnetic Stimulation (TMS) devices
It has been over a decade since the initial US Food and Drug Administration (FDA) approval of Transcranial Magnetic Stimulation (TMS). The technology was first approved for treating Major Depressive Disorder (MDD) in adults who have not responded satisfactorily to prior antidepressant medications in 2008 using the Neuronetics Neurostar System (DEN070003). Since then, refinement and optimization of TMS has paved the way to new and emerging technology that improves and broadens the clinical utility of TMS – such as pulse train protocols (e.g., iTBS and 18/20 Hz stimulation), neuronavigational systems, and electromagnetic coil technology. Alongside ongoing clinical trials, the approval of TMS therapies by the FDA has underpinned clinical adoption in the US. Here we summarize FDA regulatory milestones for TMS and provide a visual timeline of these approvals in Fig. 1.