Neurocircuit strategy to decrease cocaine cue reactivity
Our new study explores the combined efficacy of continuous theta burst stimulation (cTBS) and N-acetylcysteine (NAC) as tools to restore corticostriatal circuitry function and dampen cue-induced craving in treatment-engaged individuals with cocaine use disorder. The investigators include: Dr. Hesheng Liu, Dr. Bashar Badran, and Dr. Catherine Hubbard.
Learn more about our Center for Opioid and Cocaine Addiction: Click Here
Project Overview
The overarching goals of Project 4 are to evaluate the efficacy of combined cTBS + NAC on addiction pathophysiology in corticostriatal circuitry, and to bidirectionally translate our findings with preclinical results from Project 3. cTBS is a non-invasive brain stimulation tool that induces long-term depression (LTD) of neural excitability in the area stimulated and in monosynaptic targets. Recent data from our group demonstrates that a single dose of cTBS to the medial prefrontal cortex (mPFC) selectively decreases activity in the ventral striatum and subsequent craving (Fig. 1).
Figure 1. Interleaved TMS/MRI in cocaine users (n = 14) reveals that a single session of mPFC LTD-like cTBS specifically decreases activity in orbitofrontal cortex and ventral striatum [1].
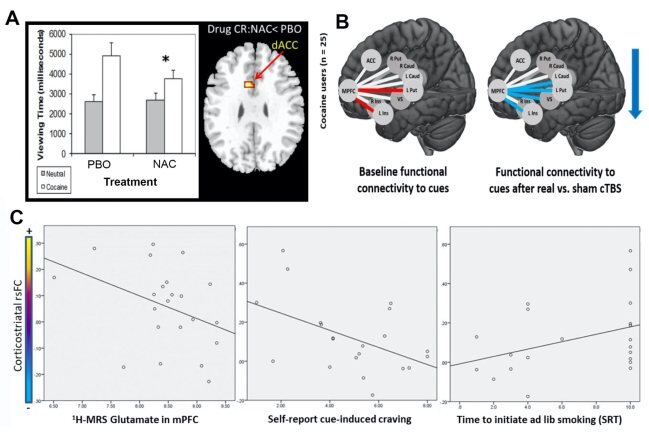
The efficacy of cTBS as a tool to modulate the striatum depends upon connectivity between the frontal cortex and the striatum. Given that cocaine users have weaker resting-state functional connectivity (rsFC) in the corticostriatal circuit, it may be beneficial to strengthen connectivity in this circuit with the cysteine pro-drug NAC. In a double-blind, placebo (PBO) controlled crossover Phase I study (Phase I) of NAC for treating cocaine dependence, our group examined the effects of NAC on gaze (viewing) time and brain reactivity to cocaine cues using functional MRI (fMRI). Compared to PBO condition and relative to neutral cues, NAC reduced gaze time to cocaine cues and attenuated drug cue-reactivity in the dACC (Fig. 2A), suggesting NAC may dampen the glutamatergic PFC projection to the accumbens. Consistent with that hypothesis, we recently found that a single dose of cTBS targeting the mPFC can attenuate functional connectivity between the mPFC and structures comprising the dorsal striatum (Fig. 2B). Moreover, our group recently demonstrated NAC increases corticostriatal rsFC in nicotine users concomitant with reducing craving and relapse (Fig. 2C). Through regulation of glial glutamate, NAC normalizes synaptic plasticity and restores the balance in corticostriatal glutamate transmission.
Figure 2. (A) N-acetylcysteine (NAC) relative to placebo (PBO) induced decreases in drug-cue reactivity and BOLD signal responses in the dorsal anterior cingulate cortex (dACC) for individuals with cocaine use disorder. NAC (2400mg/day x 3 days) reduced cue viewing (left) and drug-related responses in dACC (right) among abstinent cocaine-dependent participants (N = 11) [1]. (B) Non-treatment seeking individuals with cocaine use disorder performed a drug-cue reactivity task immediately before and after real or sham cTBS directed at the ventromedial prefrontal cortex (vmPFC). All participants received both real and sham cTBS. At baseline drug cue exposure led to an increase in functional connectivity between the vmPFC and the left putamen (t = 2.71) and left insula (t = 2.12) (Left image; red lines, Cocaine vs. Neutral cue contrast t-values; all p < 0.05)
We posit that the effects of NAC on glutamatergic homeostasis and corticostriatal circuit connectivity will enhance the efficacy of cTBS. Specifically, we seek to determine if LTD-like cTBS delivered over mPFC decreases cocaine cue reactivity in individuals with cocaine use disorder (Aim1), if NAC strengthens corticostriatal rsFC (Aim 2), and if combining cTBS + NAC has synergistic effects on reducing drug-cue reactivity and craving (Aim 3). These aims will be evaluated through a double-blind placebo-controlled study (cTBS vs. SHAM; NAC vs. PBO) in 106 cocaine dependent individuals using fMRI resting-state and a task-based drug cue-reactivity paradigm.

![Figure 1. Interleaved TMS/MRI in cocaine users (n = 14) reveals that a single session of mPFC LTD-like cTBS specifically decreases activity in orbitofrontal cortex and ventral striatum [1].](https://images.squarespace-cdn.com/content/v1/5e1fb1967993de38e958cfa5/1603386061504-FEJ4MXIDF0XNFFO00TMH/project+4-2.jpg)